Overview
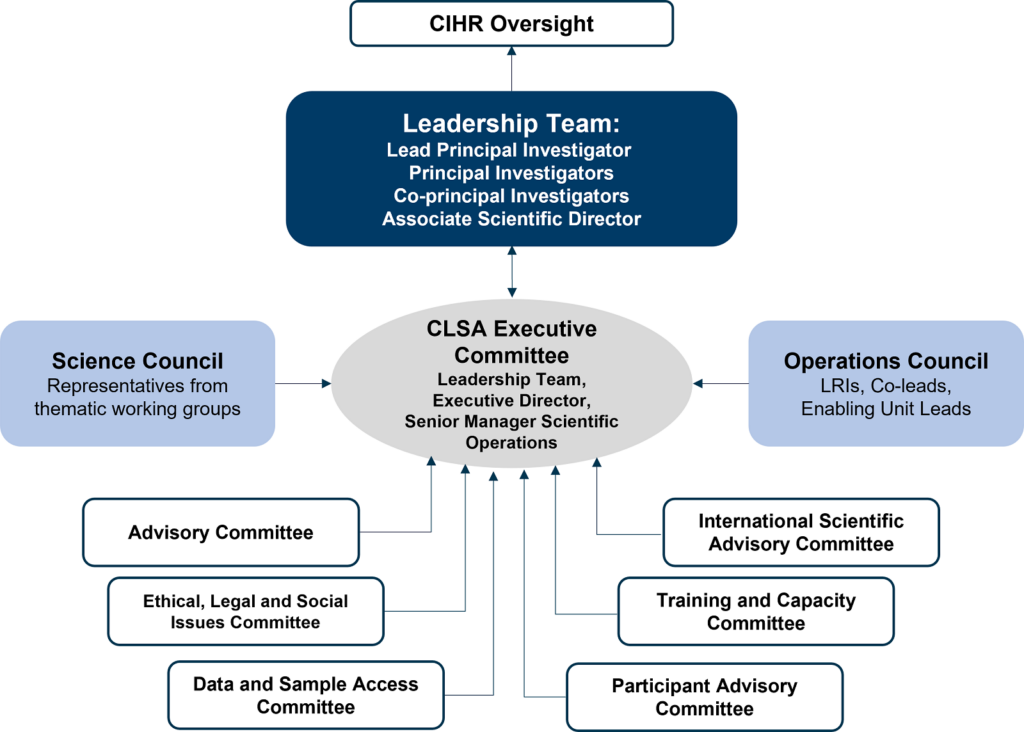
The governance and management structure supports the implementation, operation, functionality and sustainability of the CLSA research platform. It consists of two councils and six committees that report to the CLSA Executive Committee, which is composed of the CLSA’s scientific leadership team, executive director and senior manager of scientific operations. The Executive Committee is accountable to the Canadian Institutes of Health Research (CIHR).

CLSA Governance Structure
The CLSA governance structure provides robust management flexible enough for modification and growth over time, and to oversee management of scientific and succession strategies.
Oversight
Executive Committee
The CLSA Executive Committee (EC) provides strategic direction, oversight, and leadership to ensure the successful implementation of the CLSA protocol.
The EC is comprised of the CLSA’s leadership team (principal investigators, co-principal investigators and associate scientific director), executive director and senior manager of scientific operations. Meetings are held weekly.
The EC liaises with the Canadian Institutes of Health Research (CIHR) Oversight Committee and is supported by two councils and six committees (see figure above). The councils provide advice to the EC for the development of strategic scientific directions or platform policies and processes. The committees provide advice to the EC from the lens of their specific expertise and committee mandate.
Operations Council
The Operations Council (OC) provides advice on the development of strategic scientific directions, platform policies and operational processes to the CLSA Executive Committee.
The OC is composed of all enabling sites’ local principal investigators and directors.
The OC meets monthly.
Science Council
The CLSA Science Council provides advice to the Executive Committee on the development of strategic scientific directions or platform policies and processes. The Science Council is composed of working group leads and experts in the field.
The CLSA Working Groups play in important role in the creation of scientific content for the CLSA. The CLSA has the following working groups for Follow-up 4:
Social Working Group
Domains: Social networks and social support, work and retirement, stability and change of place, structural inequalities, perspectives of aging as a social process, basic social characteristics.
Lead: Denise Cloutier, Department of Geography, University of Victoria
Psychology Working Group
Domains: Cognitive, everyday competence, adaptive functioning, coping, personality, emotion, psychopathology, values, pain, sleep
Co-leads: Vanessa Taler, School of Psychology, University of Ottawa and Bruyere Research Institute
Theone Paterson, Department of Psychology, University of Victoria
Lifestyle and Nutrition Working Group
Domains: Weight fluctuations, nutritional risk, dietary supplement, physical activity, smoking, alcohol use
Lead: Isabelle Dionne, Faculty of Physical Activity Sciences, University of Sherbrooke
Health Services and Long-term Care Working Group
Domains: Continuing care, home support, long-term care institutions, acute care, primary care, administrative data sources
Lead: Andrew Costa, Department of Health Research Methods, Evidence, and Impact, McMaster University
Clinical Working Group
Domains: Cardio/Cerebrovascular (stroke, hypertension, myocardial infarction, angina); Metabolic (diabetes, hypothyroidism); Musculoskeletal (osteoporosis, osteoarthritis – hand, knee, hip); Neuropsychological (dementia, Parkinson’s disease, disability, depression); Respiratory (chronic obstructive pulmonary disease, asthma)
Lead: David Hogan, Departments of Medicine, Neurosciences, and Community Health Sciences, University of Calgary
Biology/Omics Working Group
Domains: Biological markers, genetic and epigenetic markers, clinical chemistry, quality management system
Co-leads: Cynthia Balion, Department of Pathology and Molecular Medicine, McMaster University
Brent Richards, Departments of Medicine, Human Genetics, Epidemiology & Biostatistics, McGill University
Methodology Working Group
Domains: Choosing the sample, computing sample weights, identifying (and if necessary developing) appropriate statistical methods for analysis
Lead: Edwin van den Heuvel, Department of Mathematics and Computer Science, Eindhoven University of Technology (Netherlands)
Medications Working Group
Domains: Prescription and non-prescription medications, medication list (including medication name, dosage, frequency, duration, and reason for use), medication data cleaning algorithms
Lead: Lisa Dolovich, Department of Family Medicine, McMaster University
International Scientific Advisory Committee
The CLSA International Scientific Advisory Committee provides overall advice and direction to the CLSA Executive Committee on international best practices for longitudinal research.
The Committee is composed of leading researchers in aging and health from around the world. The group meets two to three times per year.
Advisory Committee
The Advisory Committee provides overall guidance and advice to the CLSA Executive Committee on policy and promotional aspects of the study. Responsibilities include identifying and promoting opportunities for ongoing funding for the research operations. Members of the Advisory Committee are expected to advocate for the CLSA in the public and private sectors, and to establish mechanisms to ensure that the outcomes of the CLSA research are disseminated, adopted and integrated into health- and social-care policies, both economic and social.
The Advisory Committee consists of 10 to 12 members, including stakeholders from federal and provincial governments, health charities and the private sector.
Members of the Advisory Committee are selected by Executive Committee. The committee meets up to four times annually.
Data and Sample Access Committee
The Data and Sample Access Committee (DSAC) reviews applications for access to, and use of, data and/or biological samples, collected as part of the CLSA. The committee functions in accordance with the CLSA policies, guidelines and procedures for data and sample access.
The committee is composed of at least eight voting members appointed by the Executive Committee. The composition of the committee is intended to reflect the diversity of disciplines within the CLSA research program, as well as the expertise needed to review the breadth of applications.
The DSAC meets four times per year (or as needed) to review submitted applications.
Training and Capacity Committee
The CLSA is in the process of redeveloping this committee and exploring new ways to develop the next generation of researchers and highly qualified personnel.
Ethical, Legal and Social Issues Committee
The CLSA Ethical, Legal and Social Issues (ELSI) Committee provides independent advice to the CLSA Executive Committee on actions and best practices to address ethical, legal and social issues that arise or are foreseen in the planning and conduct of CLSA activities.
The ELSI Committee is composed of up to eight members who are appointed and renewed by the CLSA Executive Committee in consultation with the Committee chair. Members are sought from various relevant sectors and professions. Members serve a three-year term, with the possibility of two re-appointments.
Participant Advisory Committee
The CLSA is in the process of establishing a Participant Advisory Committee and exploring new ways of reaching participants.